Abstract
Introduction: MF is a Philadelphia-negative myeloproliferative neoplasm (Ph-negative MPN) associated with driver mutations in the JAK-STAT pathway (e.g. JAK2, CALR, MPL) and other mutations in genes that lead to epigenetic changes and altered RNA splicing (e.g. TET2, SRSF2, ASXL1, EZH2). The RAS-signaling pathway is frequently altered in acute myeloid leukemia (AML) and other myeloid malignancies, but few studies have evaluated the prevalence of such mutations in patients with MF. We sought to describe the frequency and clinical features of RAS mutations in patients with MF.
Methods: We analyzed next-generation sequencing data from 723 patients with a diagnosis of primary MF (N=520), post-PV MF (N=119) and post-ET MF (N=84). Sequencing was performed with either paired tumor-normal whole exome sequencing (WES; N=56) or selected gene panel for genes associated with myeloid malignancies (N=667). The following 16 genes were analyzed in all 723 patients and were considered as the common denominator for analysis: ASXL1, CALR, DNMT3A, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NRAS, RUNX1, TET2, TP53, WT1. RAS mutations were considered as oncogenic mutations in NRAS and/or KRAS. Molecular high risk (MHR) mutations were considered as mutations in any one of the 4 genes: ASXL1, EZH2, IDH1, IDH2 (SRSF2 mutations were not included since they were not evaluated in all cases). Odds ratio (OR) and P-values were estimated using Fisher's exact test in pairwise comparisons among genetic features, and P-values were adjusted for multiple comparisons using the Benjamini-Hochberg procedure, with significant Q-values considered as those <0.15. Overall survival (OS) was estimated using the Kaplan-Meier method, and defined as the time from the date of sample collection for mutation analysis until death from any cause, with patients alive at last follow-up censored. Cumulative incidence of AML transformation (CI-AML) was defined from the time of mutation analysis until evolution to AML, considering death without AML transformation as a competing risk. A Cox proportional hazards model was fitted to determine the hazard ratio (HR) of independent covariates associated with the dependent variable OS.
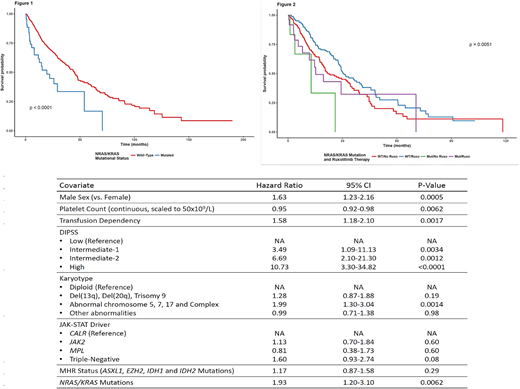
Results: Mutations in RAS genes were found in 44 patients (6.1%; 95% confidence interval [CI] 4.5-8.1%). There were 32 cases with NRAS mutations (4.4%; 95% CI 3-6.2%), 15 cases with KRAS mutations (2%; 95% CI 1.2-3.4%), and there were 3 cases with mutations in both genes (0.4%; 95% CI 0.1-1.3%). JAK-STAT driver genes found in the cohort included JAK2 (N=486), CALR (N=94), MPL (N=35) and Triple-negative status (N=108), and there was no difference in the distribution of JAK-STAT driver genes among patients with NRAS/KRAS mutations (p=0.96). Patients with NRAS/KRAS mutations had a higher number of non-driver mutations (median 3 vs 1; p=8.5e-20), higher white blood cell counts (median 15.1x109/L vs 10.5x109/L, p=0.02) and more frequently harbored ASXL1 mutations (OR 3.00, q=0.01), EZH2 mutations (OR 3.40, q=0.11) and MHR mutations (OR 3.13, q=0.004). There was a negative association of NRAS/KRAS mutations with del(20q) changes in karyotype (OR <0.001, q=0.06). After a median follow-up of 30 months and 439 events, OS was significantly reduced in patients harboring NRAS/KRAS mutations (median 19.5 months vs. 44.6 months, HR 2.95, p-value=5.99e-06; figure 1). The presence of NRAS/KRAS mutations was also associated with a higher CI-AML (4 years 18.4% vs. 14%, p-value=0.03). In a multivariate Cox model, NRAS/KRAS mutations were associated with worse OS after adjusting for other prognostic variables in MF (Table 1), including Dynamic International Prognostic Score System (DIPSS), karyotype and presence of MHR mutations. In a subset analysis of 396 patients with Int-2/High risk DIPSS, there were 202 patients who were treated with ruxolitinib. NRAS/KRAS mutated patients who received ruxolitinib (N=24) had superior OS than patients who did not receive the drug, but still inferior when compared to patients with wild-type NRAS/KRAS (Figure 2).
Conclusion: Patients with a diagnosis of MF who harbor NRAS/KRAS mutations comprise a high-risk subgroup with poor outcomes. NRAS/KRAS mutational status should be evaluated in future prognostic models in MF. The efficacy of JAK2 inhibitors needs to be further studied in this subgroup of patients, as well as the possibility of targeting the RAS pathway with MEK inhibitors.
Rampal:Incyte: Honoraria, Research Funding; Jazz: Consultancy, Honoraria; Celgene: Honoraria; Stemline: Research Funding; Constellation: Research Funding. Verstovsek:Italfarmaco: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.